Dental Offices and OSHA: Cross-Contamination Standards
Cross-contamination standards are a top concern for dental offices and OSHA. Hygienists and other patient-facing staff regularly encounter hazards that could lead to outbreaks. Following the proper guidance and keeping detailed logs can minimize risks and protect your staff.
OSHA Cross-Contamination Standards for Dental Practice
OSHA does not have any specific standards for dental practices. Instead, dental offices are instructed to follow the general clause and subject-specific recommendations that might pertain to their office. OSHA points to CDC guidance on cross-contamination standards that can apply to the work that dental practitioners do every single day.
Cross-contamination happens whenever a worker picks up a contaminant during their daily activities and spreads it around their workplace or even their home. In dental offices, the obvious contaminant risks come from biological material. Patient saliva, mucus, blood, and other bodily fluids pose a consistent risk.
The best standard in place to prevent cross-contamination from this is the CDC’s Guidelines for Infection Control in Dental Health-Care Settings from 2003.
There are eight categories of recommendations.
- Personal health elements: This covers creating a written health program for your team. This should cover policies, procedures, and guidelines for exposure prevention, post-exposure management, immunizations, and work-related illnesses and restrictions.
- Bloodborne pathogen transmission prevention: These recommendations cover the standard procedure for all patient encounters and sharps management. This section also covers workplace design and instrument use to minimize risks.
- Hand hygiene: The CDC provides specific requirements for hand washing, glove use, disposal, and lotions to minimize dry skin. It also recommends banning artificial fingernails and hand or nail jewelry if they interfere with the ability to wear gloves.
- Personal protective equipment: This section recommends the use of masks, protective eyewear and clothing, gloves, and face shields. Glove use is recommended whenever the potential for contacting blood, saliva, OPIM, or mucous membranes exists. It also covers glove disposal to minimize cross-contamination risks.
- Contact dermatitis and latex hypersensitivity: All patients should be screened for sensitivity, and emergency treatment kits with latex-free products should always be available. Patients with latex sensitivity should be kept in designated safe zones to minimize the risk of an allergic reaction.

- Sterilization and disinfection of patient care items: This covers sterilizing FDA-cleared medical devices, decontaminating work areas, instrument processing areas, and sanitizing unwrapped instruments. This series of recommendations is one of the most extensive, and practices will focus the brunt of their training and written policies here.
- Environmental infection control: Environmental infection control covers housekeeping and surface cleanliness, as well as furnishing and carpets. It also covers cleaning spills from blood or bodily fluids and using EPA-registered hospital disinfectant products.
- Dental unit water lines, quality, and biofilm: This section covers the water quality of the dental practice to meet EPA standards for drinking water and dental treatment output water. It also recommends discharging water and air for a minimum of 20 to 30 seconds after each patient when using any device connected to a dental water system that enters the patient’s mouth. There is also a series of recommendations for when boil water advisories are in effect in the practice area.
- Special considerations: This is the longest series of recommendations covering many stand-alone subjects. This includes dental handpieces, radiology, aseptic techniques, single-use devices, preprocedural mouth rinses, oral surgical procedures, and handling of biopsy specimens and extracted teeth. Also covered under this section are dental laboratories, lasers, and surgical smoke, mycobacterium tuberculosis, Creutzfeldt-Jakob disease, and program evaluation.
These are the primary areas that dental practices need to follow to minimize their risk of cross-contamination. Of course, things have changed since 2003 when these recommendations were initially made. One new cross-contamination risk that dental practices must contend with is COVID-19.
Managing COVID-19 Cross-Contamination Risk
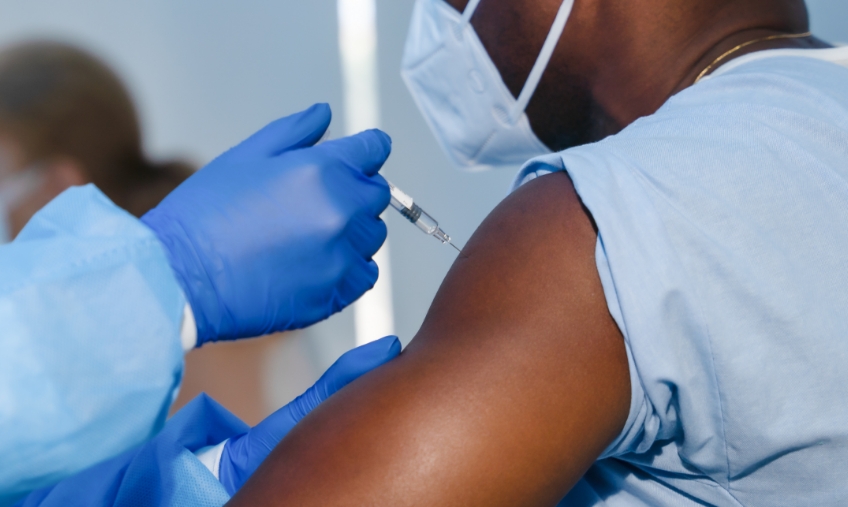
One of the most prevalent, and recent, concerns regarding cross-contamination came from COVID-19. The virus placed dental offices at elevated risk of worker illnesses and outbreaks. While OSHA guidelines have been relaxed since the start of the pandemic, there are still some things that all practices should do to minimize the risk of an outbreak in their workplace.
- Vaccination: OSHA recommends vaccination for all eligible individuals and encourages employers to provide time off for individuals to get vaccinations and recover from them.
- Mask wearing: OSHA recommends that individuals wear masks regardless of vaccination status in areas of substantial or high transmission. Exposed individuals should also wear a mask in public for 14 days after exposure or until they test negative.
- Social distancing: Particles containing the virus can travel more than six feet. OSHA encourages proper social distancing when possible, like in waiting areas. Effective room ventilation is also critical for minimizing the cross-contamination risk.
Many cross-contamination controls for COVID-19 are the same as any other cross-contamination control methods. Proper sanitization, disposal, protective equipment, and management can help minimize the risk. Record-keeping is another important part of managing cross-contamination.
Record Keeping Minimizing Cross-Contamination
Regular logging can ensure that contaminated instruments or workspaces are not used again without sanitization. Team members should be keeping track of when items are sterilized, for how long, and with what equipment. That makes it far easier to track down an issue if one occurs. For example, you may discover that a specific autoclave is not working at the end of the day. With proper records, the dental office can quickly tell what other equipment is affected so they can fix the problem and prevent cross-contamination.
OSHA Training and Support
Training is a huge part of meeting OSHA’s cross-contamination standards. Your team must understand a wide range of policies and procedures to minimize the risk to them and the public. Regular training will ensure they can meet the standards to prevent outbreaks.
SPEAR NAVIGATOR
Transform how your practice runs by engaging the team through
coaching and training
A guided path to excellence through structured coaching and self-guided resources that will align your team, streamline processes and drive growth. Transform your practice by implementing Spear’s proven playbooks for developing and retaining a high-performing dental team.

By: Spear Team
Date: February 2, 2023
Featured Digest articles
Insights and advice from Spear Faculty and industry experts