Should Dentists Use Kovanaze Nasal Spray?
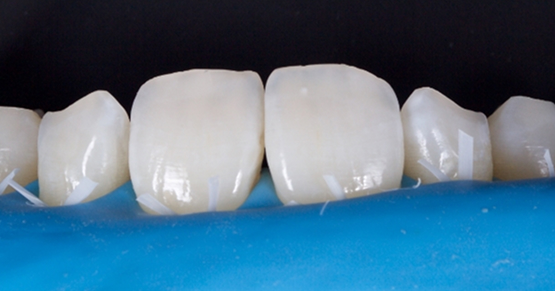
Wouldn’t it be nice to deliver a set of eight upper veneers and ask the patient to smile? Wouldn’t it been even better if you and the patient could evaluate the restorations without numbing them?
In most situations prior to removing temporaries, we need to anesthetize our patients to prevent patient discomfort. It is very challenging to evaluate the final restorations after anesthetizing the patient because patients cannot move their upper lip. This prevents us from being able to evaluate proper incisal edge position and lip mobility.
Recently you may have read that the FDA recently approved Kovanaze Nasal Spray for use as dental anesthesia. Kovanaze Nasal Spray is expected to be available this fall and should serve as an excellent adjunct in dentistry. Practitioners are always trying to create a painless experience for patients, and injections often create stress not only for the patient, but also for many dentists. The goal and biggest benefit of this anesthetic delivery system is it serves to help eliminate this concern in the anterior maxillary arch. With Kovanaze, you can achieve pulpal anesthesia without a needle while maintaining lip mobility and feeling.
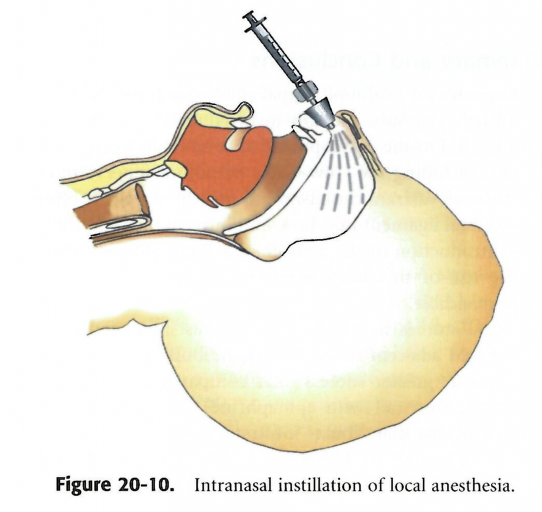
According to Dr. Stanely Malamed, absorption of drugs through the nasal mucosa to achieve the desired effect has long been established in medical procedures. The nares are highly vascularized, so most drugs can be absorbed rapidly and distributed systemically.1 Intranasal drugs are used primarily in otorhinolaryngology practices, critical care medicine and pediatric dentistry. Tetracaine is an ester local anesthetic that blocks the sodium ion channels required for the initiation and conduction of neuronal impulses. Tetracaine has been used by otorhinolaryngologists to achieve anesthesia prior to surgical manipulation of the nose.1
Patient reports indicate that their upper teeth felt numb. It was from these findings that companies attempted to find a dental application for tetracaine. More than 10 years ago, St. Renatus L.L.C. began to develop a product for dental application. Kovanaze was designed “to be a catalyst for enhanced patient comfort and care and … (to) make the dental experience for patients and dentists alike more comfortable and less stressful.”2 The pharmaceutical company was able to combine oxymetazoline to tetracaine, which caused vasoconstriction of dilated arterioles thus reducing nasal blood flow. This in turn improved the efficacy of the anesthetic.
The efficacy and safety of Kovanaze has been evaluated in three double-blind, multicenter, parallel group clinical studies where a routine dental restoration was performed. The first study had 110 patients who were administered either Kovanaze (n=44), tetracaine alone (n=44) or a placebo (n=22). Of the participants who received Kovanaze, 84 percent were able to complete the dental procedure without rescue medication – injected anesthesia – compared to 27 percent who received tetracaine alone. This study demonstrated the efficacy of Kovanaze and the need for oxymetazoline as a vasoconstrictor. Importantly, procedures on first premolar to first premolar (teeth #5-#12) had a 96 percent success rate compared to only a 63 percent for the second premolars (teeth #4 and #13).
The second clinical study had 150 adult patients who either received two or three intranasal sprays of Kovanaze(n=100) or a placebo (n=50). There was an 88 percent success rate in completing the dental procedure without rescue medication in the group receiving Kovanaze, compared to 28 percent who received the placebo. Again, the success rate was higher for anterior teeth versus lower second premolar teeth (96 percent versus 64 percent). The final clinical study measured efficacy and safety in pediatric patients ages 3-17. A total of 90 patients received either three intranasal sprays of Kovanaze (n=60) or a placebo (n=30). Children receiving Kovanaze were more likely to complete a dental procedure without rescue medication compared to those children receiving the placebo (90 percent versus 40 percent). As a result of these three industry sponsored clinical studies, the FDA approved Kovanaze for dental applications.
There are several contraindications for the administration of Kovanaze. Patients on MAOIs, nonselective beta-adrenergic antagonists, or tricyclic antidepressants may cause hypertension when combined with Kovanaze. If these medications cannot be discontinued, an alternative anesthetic agent should be used. Hypertension has been found to occur with agents containing oxymetazoline (like Afrin). Kovanaze should not be used with other intranasal products because oxymetazoline can slow the rate of uptake, but it does not affect the extent of absorption. Patients with hepatic disease and pseudocholinesterase deficiency should be closely monitored for signs of local anesthesia toxicity as they are at a greater risk of developing toxic plasma concentrations since they are less able to metabolize local anesthetics.
Lastly, patients should avoid agents that can cause methemoglobinemia (decreased oxygen-carrying ability of red blood cells) such as sulfonamides, acetaminophen, aniline dyes, benzocaine, chloroquine, dapsone, nitrates, phenobarbital, quinine and other similar medications. When used with Kovanaze, these have an additive effect. A full list can be found on the product packaging. Common side effects of Kovanaze are rhinorrhea, nasal congestion, mild nose bleeds, dizziness, and/or a sensation of difficulty in swallowing. Patients should be informed of the likelihood of these expected side effects. Side effects typically resolve the same day Kovanaze is administered.
Kovanaze will come supplied as pre-filled, single-use sprayers containing a clear aqueous solution of 30mg/mL of tetracaine hydrochloride and 0.5 mg/mL of oxymetazoline hydrochloride. Each sprayer will deliver 0.2 mL per application on the ipsilateral (same) side of the dental procedure. For adults 18 years and older, two sprays should be used four to five minutes apart. One additional spray can be used 10 minutes later if the patient does not have profound anesthesia. Children who weigh approximately 90 pounds or more also can receive two sprays four to five minutes apart. It is not indicated for use when a child is less than 90 pounds. It is expected to be packaged this fall in boxes of 30 sprayers. It will need to be refrigerated and must be discarded if not used within five days of being left out at room temperature.2

Kovanaze will likely be a very useful and beneficial adjunct in dentistry given that patients can be anesthetized for simple operative restorations and fixed restorations. I anticipate that one of the greatest benefits in the application will be in cosmetic dentistry since the lip will not be anesthetized. Both the patient and practitioner will be able to evaluate incisal edge position, patients smile, and lips at rest of their mock-ups, temporaries, and final restorations. This alone can save time and improve patient satisfaction. The notion of being able to provide a more pleasant experience for our patients makes this product one that I am anxiously excited to make part of my practice.
Look for packaging to be available in the fall at the ADA convention in Denver, Colo.
For more information go to http://st-renatus.com/.
Andrew Cohen, D.M.D., Spear Visiting Faculty and Contributing Author
References
“Kovanaze Is Now Approved by the FDA; Kovanaze Trade Package Insert.” St. Renatus LLC. N.p., n.d. Web. 25 July 2016.
Malamed, Stanely. Handbook of Local Anesthesia, Mosby. 6th Edition, 2013.
SPEAR campus
Hands-On Learning in Spear Workshops
With enhanced safety and sterilization measures in place, the Spear Campus is now reopened for hands-on clinical CE workshops. As you consider a trip to Scottsdale, please visit our campus page for more details, including information on instructors, CE curricula and dates that will work for your schedule.

By: Andrew Cohen
Date: September 13, 2016
Featured Digest articles
Insights and advice from Spear Faculty and industry experts